Pooling data for primary total knee implants across national registries: is the same implant used in multiple registries and for the same patient group? An observational study
April 2025: Publicatie Acta Orthopaedica
Pooling data for primary total knee implants across national registries: is the same implant used in multiple registries and for the same patient group? An observational study
LA Hoogervorst, RGHH Nelissen, LN van Steenbergen, AB Pedersen, EB Kristiansen, M Lindberg-Larsen, M Torre, E Ciminello, R Valentini, AW Grimberg, Y Wu, PJ Marang-van de Mheen.
Europese registerdata: Wordt dezelfde knieprothese in meerdere landen en voor vergelijkbare patiënten gebruikt?
Het combineren van gegevens over totale knieprothesen uit meerdere registers kan helpen om problemen sneller te signaleren. Dit werkt alleen als dezelfde prothese in meerdere registers voorkomt en wordt geplaatst bij vergelijkbare patiënten, omdat patiëntenkenmerken de resultaten van een knieprothese kunnen beïnvloeden. Voor deze studie zijn de gegevens van vier nationale registers (Denemarken, Duitsland, Nederland en Italië) bekeken om te bepalen of specifieke knieprothesen in meerdere registers voorkomen en of ze worden gebruikt bij vergelijkbare patiënten, op basis van leeftijd, BMI, geslacht en diagnose. Alle primaire totale knieprothesen geregistreerd in 2020 en 2021 werden meegenomen. Een prothese wordt als dezelfde beschouwd als de combinatie van merknaam van femur- en tibiacomponent, fixatie, congruentie, mobile bearing en patellagebruik overeenkwam. Voor prothesen die in ten minste twee registers minstens 100 keer voorkwamen, zijn patiëntkenmerken statistisch en klinisch vergeleken. Een klinisch relevant verschil werd gedefinieerd als minimaal 10% verschil in het percentage mannen en de diagnose artrose, of minimaal 5 jaar verschil in leeftijd en 5 punten in BMI.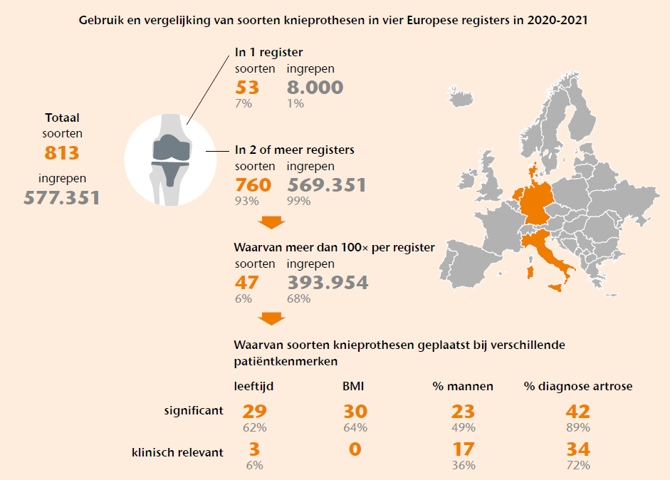
Conclusie
Veel knieprothesen komen in meerdere registers voor, waardoor gegevens gecombineerd zouden kunnen worden om problemen sneller te detecteren. Doordat deze prothesen vaak bij verschillende patiëntgroepen worden geplaatst, is een betrouwbare vergelijking van resultaten tussen registers echter lastig. Dit betekent dat resultaten over totale knieprothesen op basis van gecombineerde gegevens uit meerdere registers met voorzichtigheid moeten worden geïnterpreteerd.

